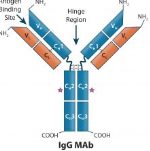
The eagerly awaited Part 2 of our review of bispecific antibodies (bsAbs) entitled “Therapeutic IgG-Like Bispecific Antibodies-Modular Versatility and Manufacturing” appears in the January 2018 issue of BioProcess International.
In this follow-up to Part 1, which highlighted the characteristics of bsAbs, we describe in detail upstream and downstream manufacturing considerations when producing bsAbs and how these engineered mAbs are used as therapeutics. Of particular relevance to the work of our client companies is our focus on optimization of culture conditions and alternative chromatographic methods for product capture.
Click to read the article entitled, “Therapeutic IgG-Like Bispecific Antibodies-Modular Versatility and Manufacturing”, authored by Jennifer Bratt, Angela Linderholm, Bryan Monroe and Steven Chamow in BioProcess International, 2018, 16, 40-49.
Chamow & Associates assists biotechnology companies in producing novel mAb therapies for clinical testing and welcomes your inquiry.