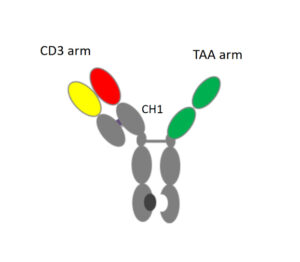
Monoclonal antibodies (mAbs) are remarkable proteins. Representing more than 50 FDA approved products for a variety medical indications, mAbs are the fastest growing class of new biologics today. Produced naturally by immune cells, these protein molecules function as the first line of defense for our immune systems to control infection and cancer–to eliminate invasive agents by binding to molecular targets.
Since approval in 1986 of the first therapeutic mAb Orthoclone OKT3 (muromonab-CD3), the field of mAb development has exploded. Starting in 2010, a series of articles entitled, ‘Antibodies to Watch’ were created to inform on events in mAb therapeutic development.
We highlight the most recent of this series, “Antibodies to Watch in 2017” by Janice M. Reichert, as she elegantly describes the current crop of antibodies undergoing clinical trials and pending approvals. Dr. Reichert highlights 5 mAbs for cancer and 15 mAbs for non-cancer indications
Click to read “Antibodies to Watch in 2017” by Janice M. Reichert