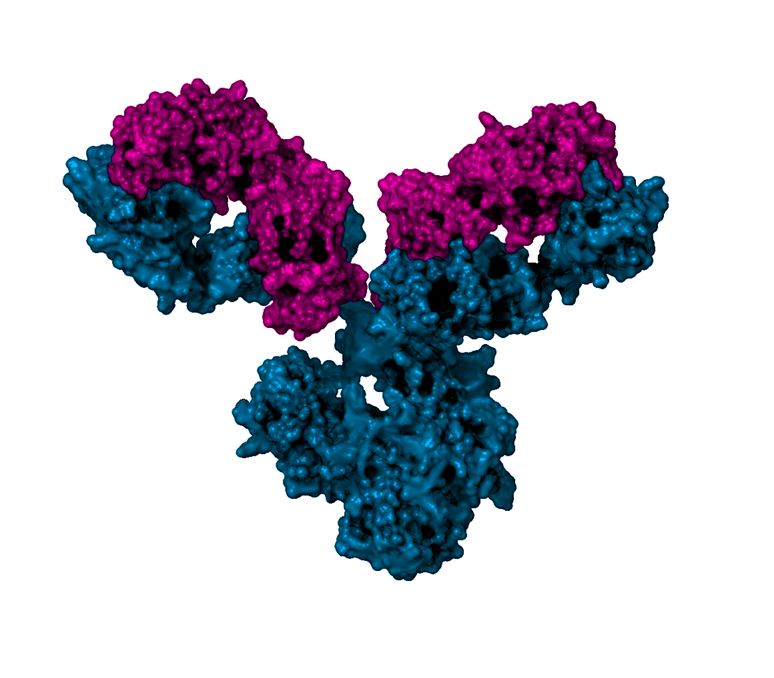
A principal reason for why the manufacture of biologics is difficult is the structural complexity of the recombinant glycoproteins that are being produced. As biologics production moves from research and development to large-scale clinical and, ultimately, commercial production, critical variables–both upstream and downstream–must be controlled to ensure reproducibility of product. One variable is the product’s charge variant profile. Stanley Chung from Northeastern University, working in collaboration with Jun Tian and colleagues at Bristol-Myers Squibb, investigated effects of four factors [temperature, iron concentration, media age, and antioxidant (rosmarinic acid) concentration] that can impact the charge profile of IgG1 monoclonal antibodies expressed in CHO cells during production. Cell culture process conditions resulting in IgG1 containing higher acidic peaks correlated with elevated supernatant peroxide concentration, intracellular reactive oxygen species or both. Changes in glycation level were the primary cause of the charge heterogeneity, and supernatant peroxide was found to positivity correlate with glycation levels. This work highlights the importance of modeling cell culture oxidative stress in order to control charge variants.
The findings are reported in “Modulating cell culture oxidative stress reduces protein glycation and acidic charge variant formation”, mAbs, 11:1, 205-216 (2019), DOI: 10.1080/19420862.2018.1537533.
Chamow & Associates assists companies to develop biologics for clinical testing and welcomes your inquiry.