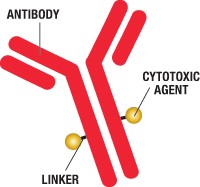
We report on antibody drug conjugates, another exciting drug class that is proving to be an important tool in the fight against cancer. Leukemias, lymphomas, and myelomas are among the most prevalent and difficult cancers to treat. Challenges include identification specific targets and delivery methods. These cancers also have significant percent of relapse and can develop immunity to treatments through upregulation of proteins such as of multidrug resistance protein 1 (MDR1) that acts as an efflux pump to pump foreign chemicals out of cells.
In a recently published paper by Penelope Drake and colleagues at Catalent Biologics in Emeryville, CA, the authors describe a MDR1 resistant anti-CD22 antibody-drug target conjugate using aldehyde tag technology to treat CD22 positive cancers. CD22 is a B-cell surface glycoprotein that has been clinically validated as a target for non-Hodgkins lymphoma. A number of cytotoxic agents that are anti-CD22 antibody conjugated have undergone clinical trials with some success. The Catalent group presents interesting data describing success in targeting CD22 positive xenografts and preclinical safety studies in non-human primates.
The coupling technology developed by this team, referred to as SMARTag™, is noteworthy. Using a non-cleavable linker, coupling of the maytansine toxin is site specific. The antibody is from a proprietary Chinese hamster ovary (CHO) cell line that overexpresses humanized anti-CD22 antibody as well as the human formylglycine (fGly) generating enzyme (FGE). The heavy chains of the anti-CD22 antibody have been modified with addition of a pentapeptide tag (Cys‑Thr‑Pro-Ser-Arg) at the carboxy terminus of each heavy chain. In FGE-expressing CHO cells, FGE converts the Cys residue of this sequence to fGly. Since fGly contains a reactive aldehyde, this creates one unique site for payload-linker conjugation on each heavy chain.
Click to view the article, “CAT-02-106, a site-specifically conjugated anti-CD22 antibody bearing an MDR1-resistant maytansine payload yields excellent efficacy and safety in preclinical models” by Penelope M. Drake, Adam Carlson, Jesse M. McFarland, Stefanie Bañas, Robyn M. Barfield, Wesley Zmolek, Yun Cheol Kim, Betty C.B. Huang, Romas Kudirka, and David Rabuka in Mol. Cancer Ther. 2018. Jan;17(1):161-168.
Catalent Biologics has licensed this anti-CD22 SMARTag™ mAb to Triphase Accelerator Corporation (Toronto, Canada) for clinical development. Chamow & Associates is assisting Triphase to produce this novel therapy for clinical testing and welcomes your inquiry.