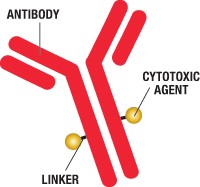
As multivalent antibodies find their way to the therapeutics market, the ability to evaluate affinity and avidity becomes a key step to their success. Separating individual binding units by cutting the molecule with enzymes is key to understanding affinity and avidity.
Proteolytic cleavage of mAbs to generate Fab and Fc fragments is typically performed using the enzyme papain. When IgG molecules are incubated with papain in the presence of a reducing agent, one or more peptide bonds in the hinge region are split, producing three fragments of similar size: two Fab fragments and one Fc fragment. Papain cleavage often requires optimization of reaction conditions and chromatographic purification of the desired Fabs. Other ways to obtain Fabs or single binding units include partial proteolysis with endoproteinase Lys-C; mild reduction of F(ab’)2 to Fab’ fragments or IgGs to half-IgGs; and recombinant expression of the light chain and the heavy Fd chain. Generally, these approaches to obtain monovalent binders are not high-throughput methods.
A new approach is available that may improve markedly on the quality and ease of generating mAb fragments. The cysteine protease gingipain K (EC 3.4.22.47) of the pathogenic anaerobe bacteria Porphyromonas gingivalis has recently become commercially available as the recombinant protease GingisKHAN™ (KGP) from Genovis AB (Lund, Sweden). GingisKHAN™ is reported to digest human IgG1s at a single site in the hinge region (KSCDK/THTCPPCP), generating a homogenous pool of intact Fabs and crystallizable (Fc) fragments.
The capability to generate a uniform pool of intact Fab and Fc fragments allows quicker measurements of binding affinities and screening of bivalent and multivalent antibodies. In a recent paper, Jorg Moelleken and colleagues from Roche Pharma Research and Early Development, Large Molecule Research, Roche Innovation Center, Germany, evaluated GingisKHAN™ as a tool to generate in-solution digestions of human IgG1s and assayed the crude mixture post-digestion for intact Fab fragments.
We suggest you take a look at their work: “GingisKHAN™ protease cleavage allows a high-throughput antibody to Fab conversion enabling direct functional assessment during lead identification of human monoclonal and bispecific IgG1 antibodies”, mAbs, 9:7, 1076-1087, DOI: 10.1080/19420862.2017.1364325.
Chamow & Associates assists biotechnology companies in producing novel therapies such as mAbs and engineered mAb derivatives for clinical testing.