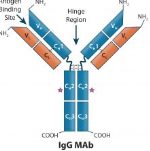
Reported on 8 March by Eric Palmer from Fierce Pharma, 2018 marks a landmark event in the global biologics supply chain—for the first time, an FDA approved biologic will be manufactured in China for the U.S. market. WuXi Biologics will produce the HIV targeting-monoclonal antibody (mAb) Trogarzo. Trogarzo was developed by Taiwan-based TaiMed Biologics and was approved by FDA in March 2018. It is a humanized mAb that binds to extracellular domain 2 of CD4 on the surface of T cells. CD4 is the receptor through which HIV infects T cells, so that by binding to CD4, the mAb prevents the virus from infecting its host. The approved mAb will be produced at WuXi’s manufacturing facility in Wuxi City near Shanghai, China, which passed a pre-approval inspection in 2017. WuXi is the first and so far only Chinese company to receive FDA clearance for a biopharmaceutical.
The manufacturing facility, completed in 2012, houses a single use disposable bioreactor and is reported by WuXi Biologics CEO Chris Chen to be a novel design for facilities of the future. Since completing that facility, WuXi has erected a $150 million, 500,000-square-foot manufacturing facility, with an additional 14 x 2,000 L single-use bioreactors for fed-batch cell culture production (2,000 L + 28,000 L for a total of 30,000L bioreactor capacity), also in WuXi City. This is now China’s largest single-use bioreactor manufacturing facility. WuXi Biologics also recently completed a cGMP production build out in Shanghai with fed batch production up to 2 x 2,000 L and perfusion up to 2 x 1000 L.
This exciting development demonstrates that biologics manufacturing in China has come of age, and with this milestone having now been reached, there will surely be more GMP facilities coming on line from other Chinese companies.
Chamow & Associates assists biotechnology companies in producing novel therapies for clinical testing at CDMOs such as WuXi Biologics and welcomes your inquiry.